Down’s syndrome is a condition caused by three copies of chromosome 21. It is also called trisomy 21. It gives characteristic dysmorphic features and has many associated conditions. The extent to which a person is affected and the related conditions they have vary between individuals.
All women are offered screening for Down’s syndrome during pregnancy. The purpose of the screening test is to decide which women should receive more invasive tests to establish a definitive diagnosis.
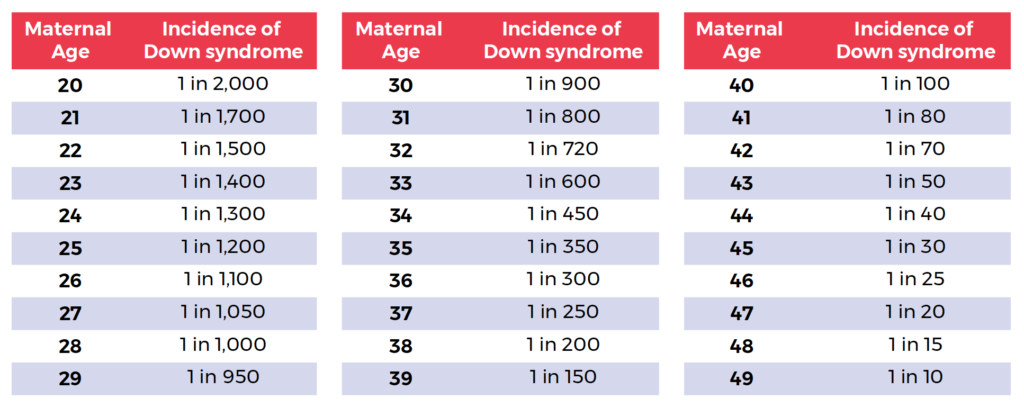
It is the choice of the woman whether to go ahead with screening. The screening tests involve taking measurements from the fetus using ultrasound, combining those measurements with the mother’s age and blood results and providing an indication of the risk of Down’s syndrome. Older mothers have a higher risk of Down’s syndrome.
Combined Test
The combined test is the first line and the most accurate screening test. It is performed between 11 and 14 weeks gestation and involves combining results from ultrasound and maternal blood tests.
Ultrasound measures nuchal translucency, which is the thickness of the back of the neck of the fetus. Down’s syndrome is one cause of a nuchal thickness greater than 6mm.
Maternal blood tests:
- Beta‑human chorionic gonadotrophin (beta-HCG) – a higher result indicates a greater risk
- Pregnancy‑associated plasma protein‑A (PAPPA) – a lower result indicates a greater risk
Triple Test
The triple test is performed between 14 and 20 weeks gestation. It only involves maternal blood tests:
- Beta-HCG – a higher result indicates greater risk
- Alpha-fetoprotein (AFP) – a lower result indicates a greater risk
- Serum oestriol (female sex hormone) – a lower result indicates a greater risk
Quadruple Test
The quadruple test is performed between 14 and 20 weeks gestation. It is identical to the triple test, but also includes maternal blood testing for inhibin-A. A higher inhibin-A indicates a greater risk.
Antenatal Testing for Down’s Syndrome
The screening tests provide a risk score for the fetus having Down’s syndrome.
- When the risk of Down’s is greater than 1 in 150 (occurs in around 5% of tested women), the woman is offered amniocentesis or chorionic villus sampling.
These tests involve taking a sample of the fetal cells to perform karyotyping for a definitive answer about Down’s:
- Chorionic villus sampling (CVS) involves an ultrasound-guided biopsy of the placental tissue. This is used when testing is done earlier in pregnancy (before 15 weeks).
- Amniocentesis involves ultrasound-guided aspiration of amniotic fluid using a needle and syringe. This is used later in pregnancy once there is enough amniotic fluid to make it safer to take a sample.
Genetic Carrier Screening
MBS Funded Genetic Carrier Screening
Starting from November 1, 2023, the Medicare Benefits Schedule (MBS) in Australia has included funding for genetic carrier screening for three specific conditions: cystic fibrosis (CF), spinal muscular atrophy (SMA), and fragile X syndrome (FXS). This initiative makes these tests free for eligible individuals and couples who are planning a pregnancy or are already pregnant, allowing them to make more informed reproductive choices without the financial burden.
Covered Conditions:
- Cystic Fibrosis (CF): A genetic disorder affecting the lungs and digestive system.
- Spinal Muscular Atrophy (SMA): A genetic disease affecting motor nerve cells in the spinal cord.
- Fragile X Syndrome (FXS): A genetic condition causing intellectual disability, behavioral and learning challenges.
This coverage ensures that reproductive carrier screening for these conditions is accessible to all eligible Australians, helping to identify carrier status and providing options such as pre-implantation genetic diagnosis or other reproductive choices if both partners are carriers.

Non-MBS Funded Genetic Carrier Screening
For those looking beyond the MBS-funded options, expanded genetic carrier screening panels are available. These can test for hundreds of genetic conditions, providing a broader assessment of genetic risks. However, these expanded panels are not covered by Medicare and can be costly, typically ranging from $450 to over $1,500. These tests are often recommended for those with specific family histories of genetic conditions or who desire a more comprehensive genetic overview.
Benefits and Considerations:
- Financial Consideration: Non-MBS funded tests can be expensive, and costs are generally out-of-pocket.
- Comprehensive Screening: These tests can identify a wider array of genetic conditions, offering more extensive information for family planning.
- Accessibility: Since these are not covered by Medicare, they are accessible primarily to those who can afford the additional expense.
Access and Process
- Consultation: Discuss with a healthcare provider to determine the need and eligibility for genetic carrier screening.
- Testing: For MBS-funded tests, the process involves a simple blood sample. Non-MBS tests follow a similar process but may involve more comprehensive panels.
- Results and Counseling: Results are discussed with a healthcare provider, often involving genetic counseling to understand the implications and options available based on the results.
These changes in funding aim to make critical genetic information accessible to more Australians, supporting better-informed decisions in family planning and pregnancy management
Non-Invasive Prenatal Testing (NIPT)
Non-Invasive Prenatal Testing (NIPT) in Australia is widely available and offered by several providers. NIPT is a blood test that screens for common chromosomal conditions in a developing fetus by analyzing cell-free fetal DNA circulating in the mother’s blood. This test is typically available from as early as the 10th week of pregnancy and is known for its high accuracy and non-invasive nature.
Key NIPT Providers and Options in Australia:
- Generation NIPT:
- Generation: Screens for common chromosomal abnormalities such as trisomy 21 (Down syndrome), trisomy 18 (Edwards syndrome), and trisomy 13 (Patau syndrome).
- Generation 46: Expands the screening to include gains and losses of all 46 chromosomes.
- Generation Plus: Includes additional detailed genomic information and is processed in an accredited laboratory in California.
- Cost: Ranges from $425 to $799 depending on the specific test chosen.
- Results Time: 3-7 business days for Generation and Generation 46, 11-15 business days for Generation Plus (Genomic Diagnostics).
- Harmony NIPT:
- Screens for the same primary trisomies (21, 18, and 13) and can optionally include testing for sex chromosome aneuploidies and microdeletions.
- Results Time: Typically available within a week.
- Harmony is known for its reliability and is widely used as a first-tier screening test (ClinicalLabs).
- Percept NIPT:
- Offered by Victorian Clinical Genetics Services (VCGS).
- Screens for conditions across all 23 chromosome pairs, providing high chance or low chance results for conditions such as Down syndrome.
- Cost: $449.
- Results Time: 3-5 working days after the laboratory receives the sample (VCGS).
General Process for NIPT:
- Consultation: Speak with a healthcare provider to determine if NIPT is suitable based on individual risk factors and preferences.
- Blood Sample Collection: A simple blood draw from the mother after 10 weeks of pregnancy.
- Laboratory Analysis: The sample is analyzed in an accredited lab to detect chromosomal abnormalities.
- Results: Delivered to the healthcare provider, who will discuss the findings with the expectant parents. Genetic counseling is often available for high-risk results.
NIPT is a personal choice and is not compulsory. It provides valuable information about the health of the fetus and can help expectant parents make informed decisions regarding further testing and pregnancy management.