In medicine, various types of clinical studies are used to investigate the safety, efficacy, and effectiveness of treatments, interventions, and healthcare practices. These studies can be broadly classified into two main categories: observational studies and experimental studies. Each type has its own strengths, weaknesses, and appropriate uses. Here is a comprehensive overview of the different types of clinical studies used in medicine:
Observational Studies
- Case Report and Case Series:
- Case Report: A detailed report of the symptoms, diagnosis, treatment, and follow-up of an individual patient. Often used to describe unusual or novel occurrences.
- Case Series: A collection of case reports involving patients who were given similar treatment. It provides insights into the clinical course and outcomes in a series of patients.
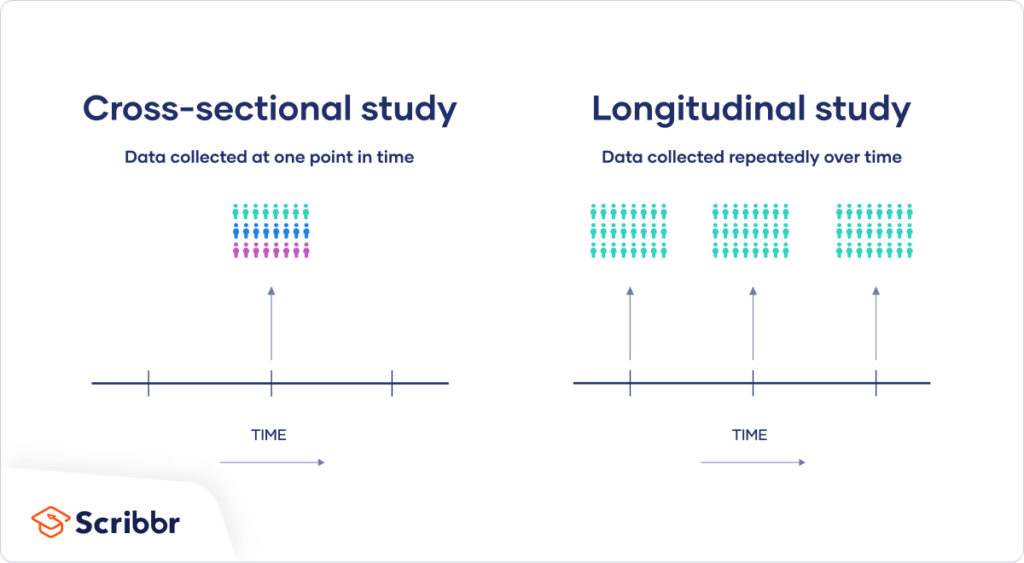
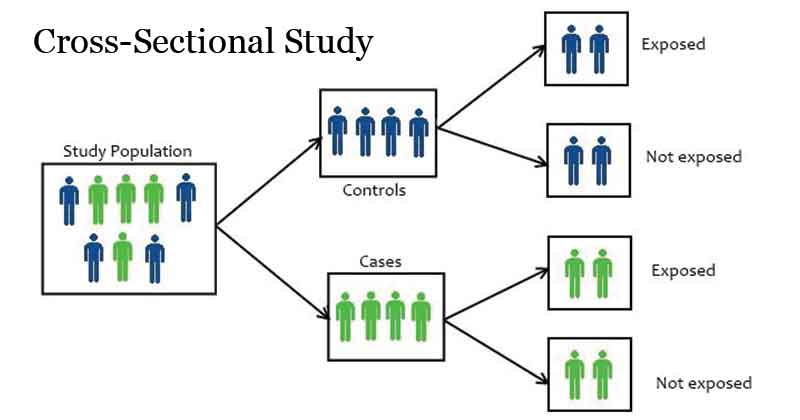
- Cross-Sectional Study:
- Descriptive study that analyzes data from a population at a single point in time.
- Useful for assessing the prevalence of health outcomes or characteristics in a population.
- Case-Control Study:
- Compares patients with a specific condition or outcome (cases) to those without the condition (controls).
- Retrospective in nature; looks back in time to determine exposure to risk factors.
- Efficient for studying rare diseases.
- Cohort Study:
- Follows a group of people (cohort) over time to see how their exposures affect their outcomes.
- Can be prospective (following individuals forward in time) or retrospective (using existing data).
- Useful for studying the incidence and natural history of diseases.
- Nested Case-Control Study:
- A type of case-control study conducted within a defined cohort.
- Cases and controls are selected from the same cohort, which reduces selection bias and improves comparability.
Experimental Studies
- Randomized Controlled Trial (RCT):
- Participants are randomly assigned to receive either the intervention being tested or a control (placebo or standard treatment).
- Considered the gold standard for testing the efficacy and safety of interventions.
- Can be blinded (participants do not know which group they are in) or double-blinded (neither participants nor researchers know).
- Non-Randomized Controlled Trial:
- Similar to RCTs, but participants are not randomly assigned to groups.
- More susceptible to selection bias and confounding factors.
- Crossover Study:
- Participants receive multiple interventions in a specific sequence.
- Each participant serves as their own control, which reduces variability.
- Suitable for conditions that are stable and interventions with temporary effects.
Other Study Designs
- Systematic Review:
- A comprehensive summary of the literature on a specific research question.
- Involves a systematic search, appraisal, and synthesis of evidence from multiple studies.
- Meta-Analysis:
- A statistical technique that combines the results of multiple studies to produce a single estimate of the effect of an intervention.
- Often conducted as part of a systematic review.
- Diagnostic Accuracy Study:
- Evaluates the accuracy of a diagnostic test by comparing it to a gold standard.
- Measures sensitivity, specificity, positive predictive value, and negative predictive value.
- Prognostic Study:
- Examines the factors that predict the outcome of patients with a particular condition.
- Often involves following a cohort of patients over time.
- Implementation Study:
- Focuses on the methods to promote the integration of research findings and evidence into healthcare practice.
- Evaluates strategies to enhance the adoption and sustainability of interventions.
- Health Services Research:
- Studies how people access healthcare services, the cost of care, and the outcomes of healthcare services.
- Often involves large databases and complex statistical methods.
Summary of Key Points
- Observational Studies: Include case reports, case series, cross-sectional studies, case-control studies, cohort studies, and nested case-control studies. These studies observe and analyze outcomes without intervention from the researchers.
- Experimental Studies: Include RCTs, non-randomized controlled trials, and crossover studies. These studies involve active intervention and manipulation of variables to assess the effects of treatments.
- Other Study Designs: Include systematic reviews, meta-analyses, diagnostic accuracy studies, prognostic studies, implementation studies, and health services research. These designs address specific research questions and provide a broader understanding of clinical and healthcare issues.
Each type of clinical study has its place in the medical research landscape, and the choice of study design depends on the research question, the nature of the disease or intervention, ethical considerations, and available resources. Understanding these various study designs helps in interpreting research findings and applying them to clinical practice.